Platelet lymphocyte ratio a diagnostic challenge in pancreatic head masses
Abstract
Background: Masses in head of pancreas may arise from Carcinoma head of pancreas or Chronic pancreatitis. There is a need for differentiating the type of pancreatic head masses with utmost priority, since it can convert a radical surgery to conventional procedure. Recent studies suggest that Platelet lymphocyte ratio (PLR) can be used as a marker for differentiating between these masses. We evaluated the role of PLR and other tumour markers with combinations in differentiating the masses in head of the pancreas.
Methods: A total of Sixty patients with mass in the head of pancreas with size 2cm or greater were included in the study, with 30 patients in the Pancreatic ductal adenocarcinoma (PDA) group and 30 patients in the Chronic Pancreatitis group. Patients with evidence of acute pancreatitis, liver cirrhosis, cholangitis and obstructive jaundice, having Lewis antigen A/B blood types, with absent tissue /cytological diagnosis were excluded.
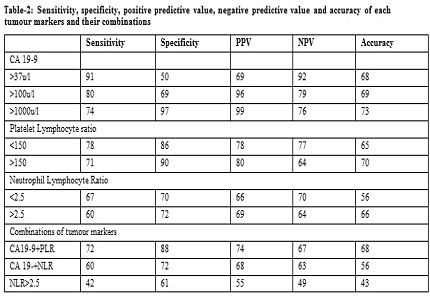
Results: Haematological parameters like platelet count, neutrophil count, lymphocyte count were not statistically significanct between the two groups, whereas, Mean CA 19-9 levels, (PLR) Platelet lymphocyte ratio levels and (NLR) Neutrophil lymphocyte ratio in (PDA) Pancreatic ductal adenocarcinoma were higher than Chronic pancreatitis group. (P<0.05) In all these patients, biomarkers such as CA 19-9, Platelet lymphocyte ratio (PLR), Neutrophil lymphocyte ratio (NLR) and different combinations of these tumour markers were calculated, and their sensitivity, specificity, (PPV) Positive predictive value, (NPV) Negative predictive value and accuracy in differentiating malignant from benign pancreatic masses were established. The diagnostic accuracy of the tumour markers and their combinations are in the following order PLR> PLR+CA 19-9> CA 19-9> CA 19-9+NLR> NLR.
Conclusion: We found that PLR is as good as tumour marker CA 19-9 both individually as well as with other tumour marker combinations in the diagnosis of pathology of pancreatic head masses. Cost effectiveness, availability, simplicity and better diagnostic accuracy made PLR better tumour marker in the current scenario.
Downloads
References
2. Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987 Oct 15;47(20):5501-3. [PubMed]
3. Wu E, Zhou S, Bhat K, Ma Q. CA 19-9 and pancreatic cancer. Clin Adv Hematol Oncol. 2013 Jan;11(1):53-5. [PubMed]
4. Bhat K, Wang F, Ma Q, Li Q, Mallik S, Hsieh TC, Wu E. Advances in biomarker research for pancreatic cancer. Curr Pharm Des. 2012;18(17):2439-51. [PubMed]
5. Duraker N, Hot S, Polat Y, Höbek A, Gençler N, Urhan N. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol. 2007 Feb 1;95(2):142-7. [PubMed]
6. Howaizi M, Abboura M, Krespine C, Sbai-Idrissi MS, Marty O, Djabbari-Sobhani M. A new cause for CA19.9 elevation: heavy tea consumption. Gut. 2003 Jun;52(6):913-4. [PubMed]
7. Ørntoft TF, Vestergaard EM, Holmes E, Jakobsen JS, Grunnet N, Mortensen M, et al. Influence of Lewis α1-3/4-L-fucosyltransferase (FUT3) gene mutations on enzyme activity, erythrocyte phenotyping, and circulating tumor marker sialyl-Lewis a levels. Journal of Biological Chemistry. 1996;271(50):32260-8. http://www.jbc.org/content/271/50/32260.long
8. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007 Apr;33(3):266-70. Epub 2006 Nov 9. [PubMed]
9. Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001 Nov 1;98(9):2720-5.
10. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010 Jan;6(1):149-63. doi: 10.2217/fon.09.136.
11. Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005 Jan 1;105(1):178-85. Epub 2004 Sep 14.
12. Miglani RK, Bhateja N, Bhat RS, Kumar KV. Diagnostic Role of Platelet Lymphocyte Ratio(PLR) in Pancreatic Head Masses. Indian J Surg. 2013 Feb;75(1):4-9. doi: 10.1007/s12262-012-0443-6. Epub 2012 Mar 27. [PubMed]
13. Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. The American Journal of Surgery. 2009;197(4):466-72. https://www.ncbi.nlm.nih.gov/pubmed/18639229
14. Bhat K, Wang F, Ma Q, Li Q, Mallik S, Hsieh TC, Wu E. Advances in biomarker research for pancreatic cancer. Curr Pharm Des. 2012;18(17):2439-51. [PubMed]
15. Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006 Nov 20;24(33):5313-27. Epub 2006 Oct 23.
16. Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008 May;143(5):658-66. PubMed PMID: 18436014. Epub 2008/04/26. eng. https://www.ncbi.nlm.nih.gov/pubmed/18436014.
17. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010 Aug;200(2):197-203. doi: 10.1016/j.amjsurg.2009.08.041. Epub 2010 Feb 1.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


















 Therapoid
Therapoid

