Clinicopathological features of Pseudocyst Pancreas and its Management: A Prospective and Retrospective Study
Koshariya M.1, Sharma A.2*, Gupta B.3, Suroshe T.4, Singour J.5, Songra M C.6
DOI: https://doi.org/10.17511/ijoso.2020.i06.01
1 Mahim Koshariya, Professor, Department of Surgery, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
2* Agam Sharma, Senior Resident, Department of Surgery, Government Medical College, Shivpuri, Madhya Pradesh, India.
3 Brahmanand Gupta, Senior Resident, Department of Surgery, Government Medical College, Shivpuri, Madhya Pradesh, India.
4 Tushar Suroshe, Senior Resident, Department of Surgery, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
5 Jaiprakash Singour, Senior Resident, Department of Surgery, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
6 Songra M C, Professor and Head of Department, Senior Resident, Department of Surgery, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
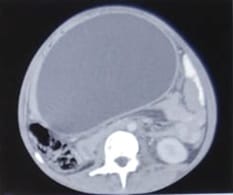
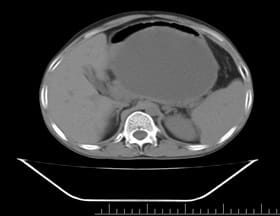
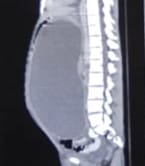
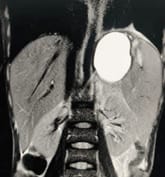
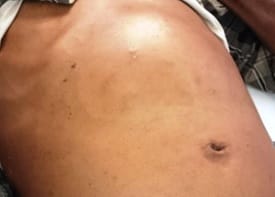
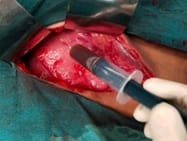
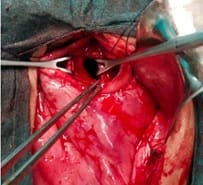
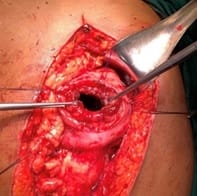
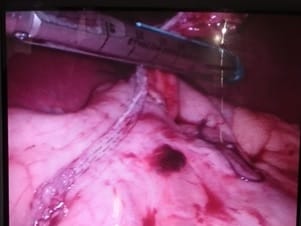
Background: Revised Atlanta Classification has veered a change in our understanding of pseudocyst pancreas which mandates renewed inquiry into pseudocysts defined as per new criteria. The present study provides an overview of experience with Pseudocyst Pancreas for over a decade. Methodology: 100 cases of pseudocysts diagnosed over the last 10 years at GMC, Bhopal, conforming to the present definition were reviewed. Cysts with the inhomogeneous collection, debris, necrosis, or any other non-liquid component, specifically in those diagnosed before 2012 were excluded. Relevant data were analyzed. Results: The majority were male (85%) in the age group of 40-50 years with alcohol-induced chronic pancreatitis (77%) being the most frequent etiology. Abdominal pain (40%), lump (30%), and abdominal tenderness (59%) were common at presentation. 58% were in the Head of the pancreas, 29% in the Neck and Body, and 13% in the Tail and surrounding areas. Mean cyst diameter was 8.6cm and volume 252cc. 85% were managed surgically and 40% of those managed conservatively also needed surgical intervention eventually due to complications. History of chronic alcoholic pancreatitis, the large size of the cyst (≥6cm and ≥60cc), and communication with the main pancreatic duct were highly predictive of surgical intervention. Conclusion: Radiological characteristics along with the clinical picture may suggest appropriate intervention. Surgery remains the principal modality of treatment, with high success rates.
Keywords: Pseudocyst, Pancreas, Pancreatitis
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Resident, Department of Surgery, Government Medical College, Shivpuri, Madhya Pradesh, India. Email: |
Koshariya M, Sharma A, Gupta B, Suroshe T, Singour J, Songra M C. Clinicopathological features of Pseudocyst Pancreas and its Management: A Prospective and Retrospective Study. Surgical Rev Int J Surg Trauma Orthoped. 2020;6(6):338-346. Available From https://surgical.medresearch.in/index.php/ijoso/article/view/200 |


 ©
©